CLINICAL DATA FOR
PHARMACEUTICAL
RESEARCH
TECHNOLOGY, STATISTICS & DATA MANAGEMENT
FOR CLINICAL TRIALS
We assist clinical trial teams to collect, manage and analyse their data. We develop our own cloud software systems, we have expertise in controlled clinical data management processes and deep know-how in statistical analysis.
CLOUD DATA COLLECTION
Our own EDC/ePRO/eCOA system, which we can quickly adapt to your needs, enrich with your requirements and which you have ready to use in the cloud. With real time monitoring tools to ensure data quality.
STATISTICAL ANALYSIS
Your statistical consultants at each stage of a trial, from inception, design and protocol to conclusive statistical analysis and reports for regulatory submission.
CLINICAL DATA MANAGEMENT
Our team of clinical data management experts can assist you in your clinical data management tasks. You can trust their experience in robust processes and compliance, and let them become part of your team to ensure data quality and minimise data risks.
CDISC
PROGRAMMING
We ensure your data are CDISC compliant, from CDASH and SDTM annotated CRFs to SDTM, ADAM and analysis scripts.
CLOUD TRIAL TOOLS
Interactive randomisation, clinical image assessment adjudication, real-time visualisation of any variable, lab data. Tools that can be quickly implemented according to your needs and efficiently integrated in your system.
REGULATORY
From an ICH compliant SAP to clinical study reports, efficacy and safety reports ready for regulatory submission.
OUR EXPERIENCE
We have worked with specialized CROs, sponsors, including most major multinational pharmaceuticals and innovative biotechs exploring new drugs, as well as with research institutes and academia, and international organizations and government bodies. We have provided technology, statistics and data management from small-scale but complex Phase I and II clinical studies, to demanding Phase III and meta-analyses for regulatory submission, to large scale, multi-centre, multi-country multilingual post marketing trials and patient registries.
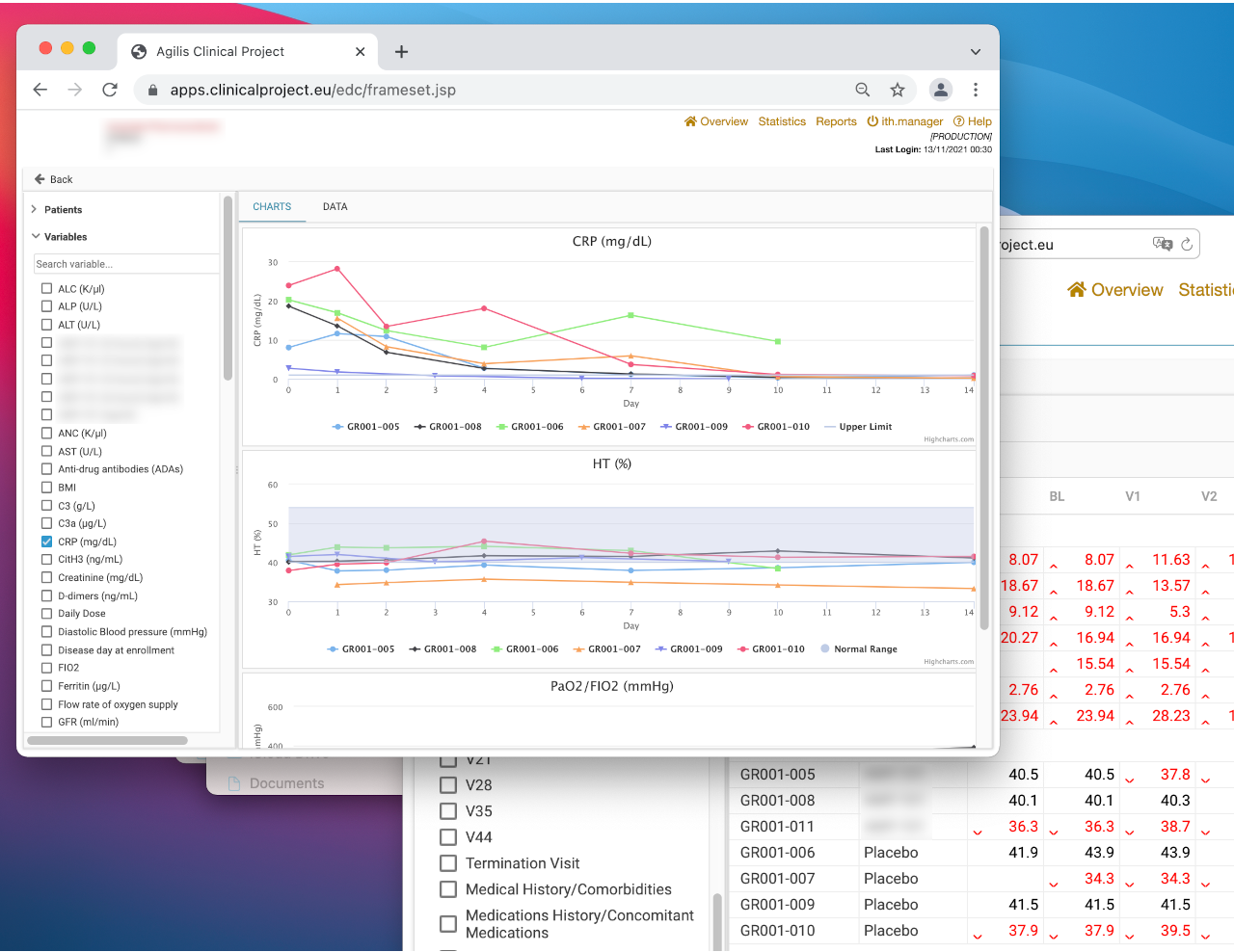
Real-Time Endpoint Charts
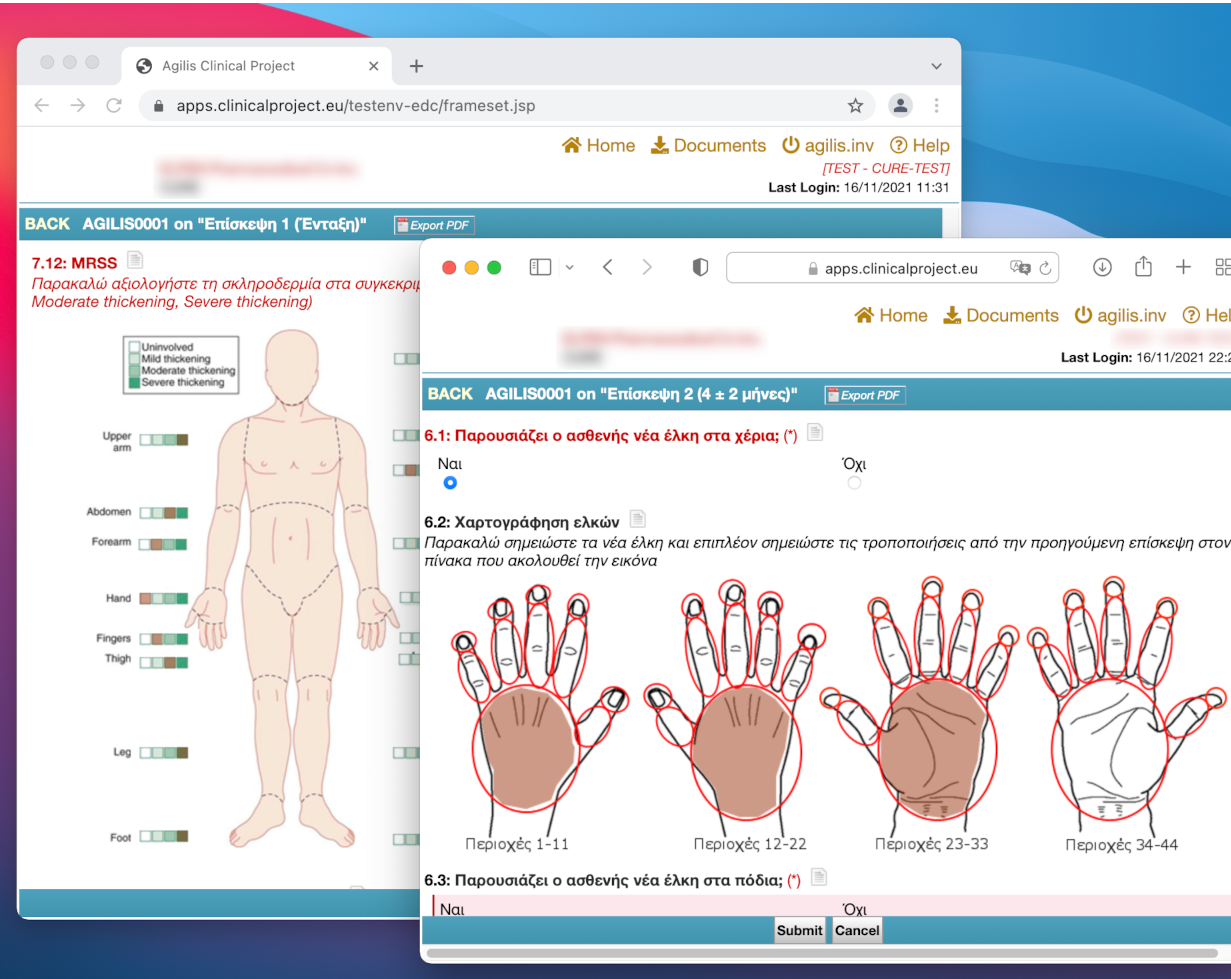
Image-based Data Entry
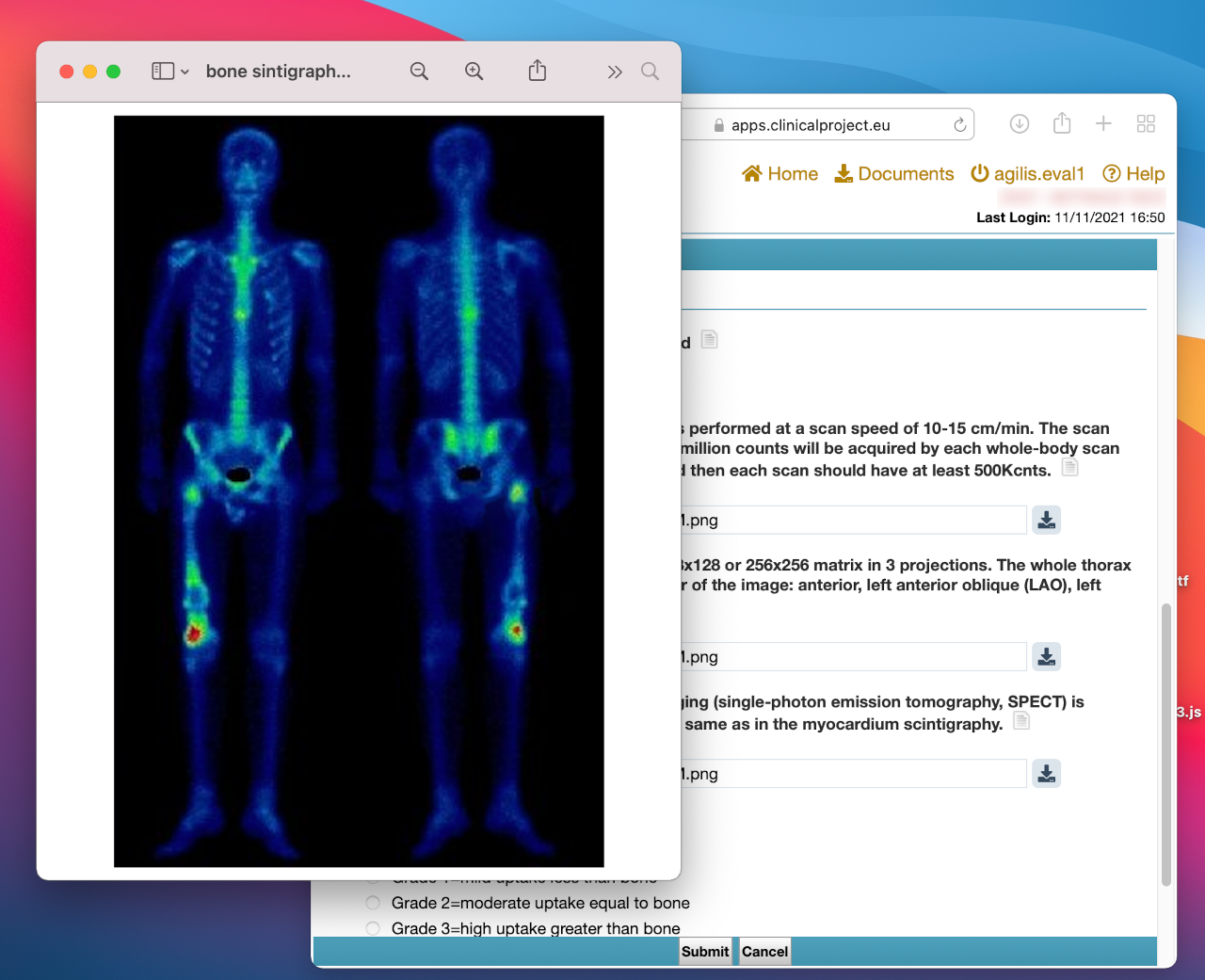
Medical Image Assessment
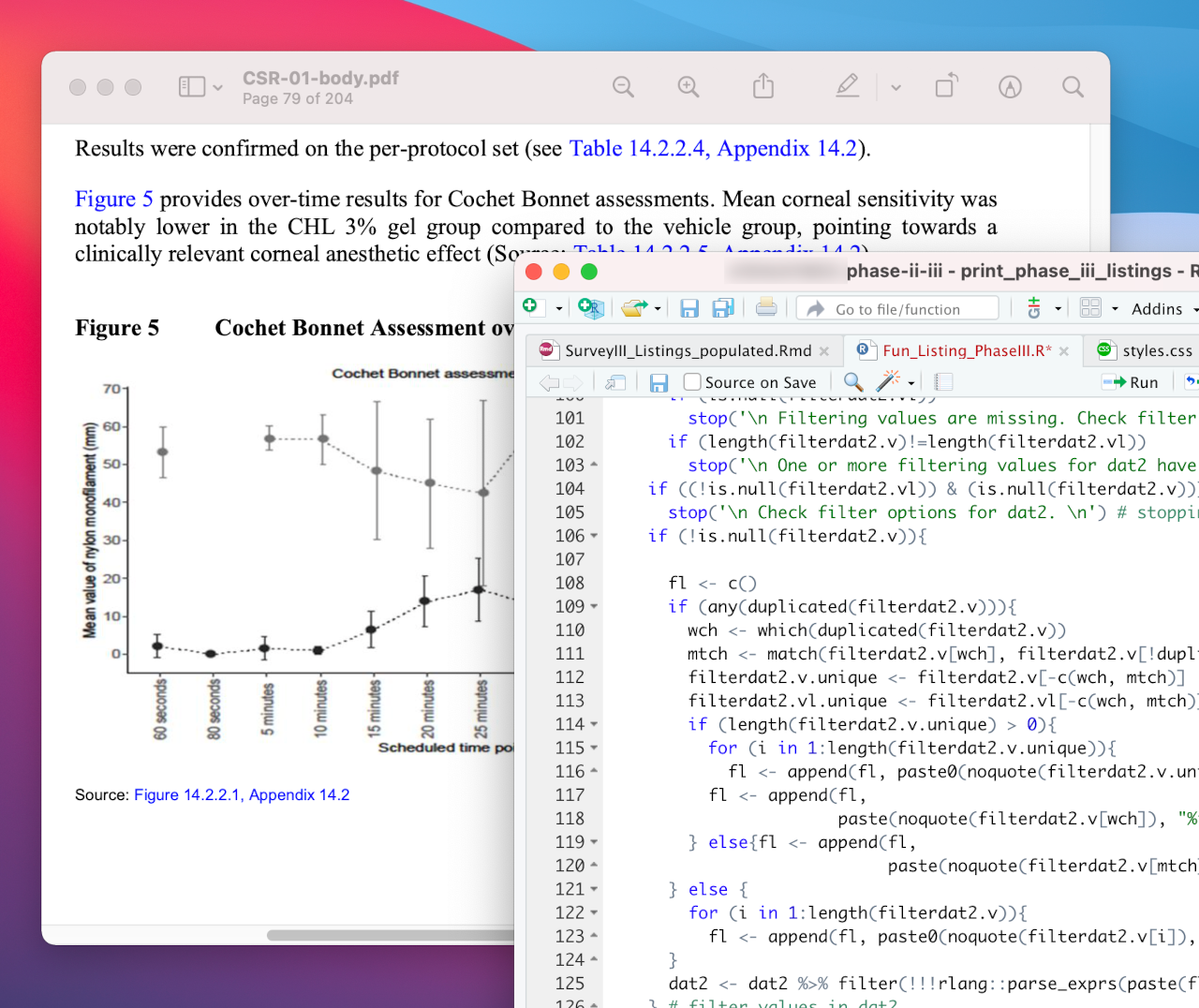
Meta-analysis of pooled data
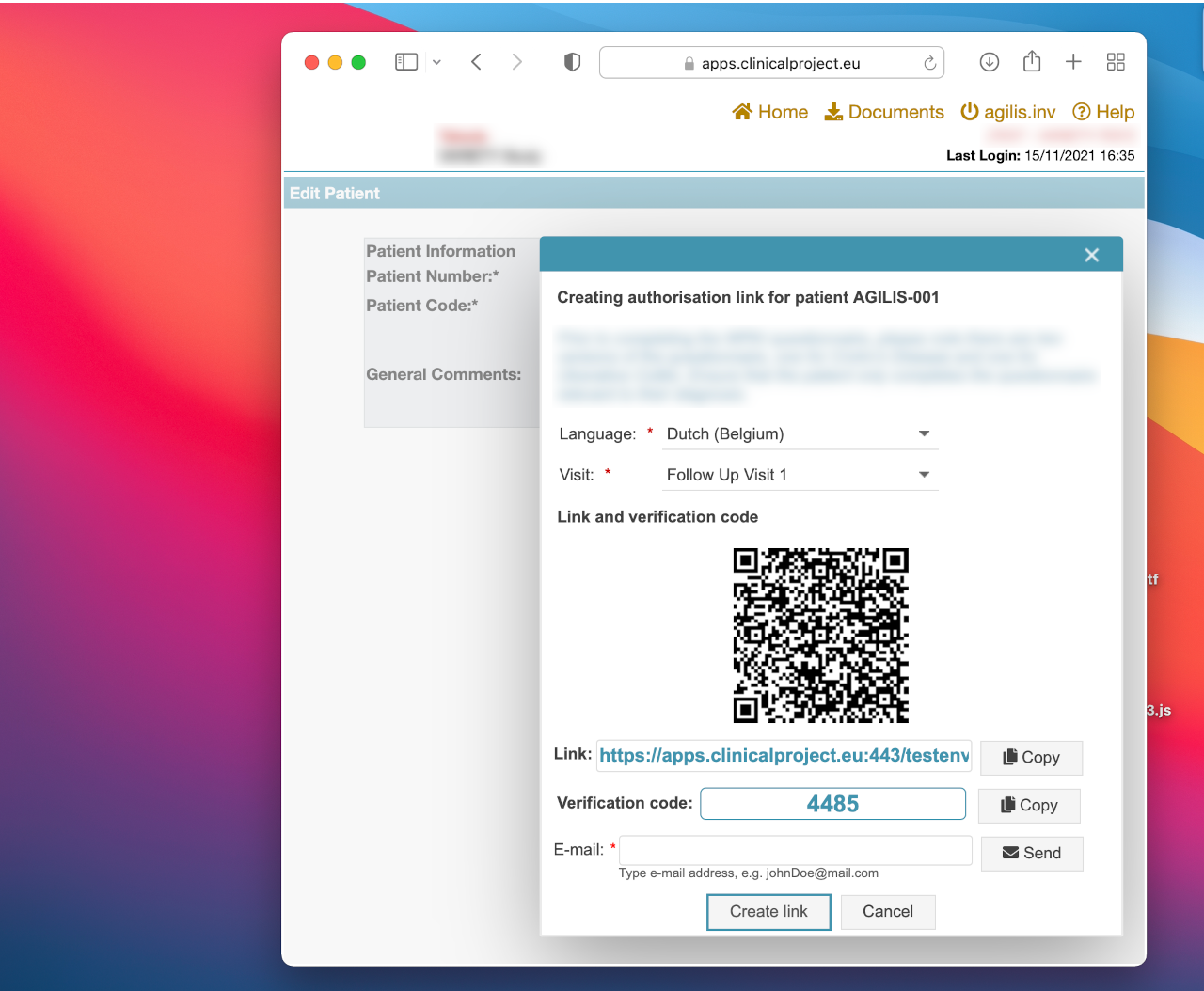
Dynamic delivery of PROs
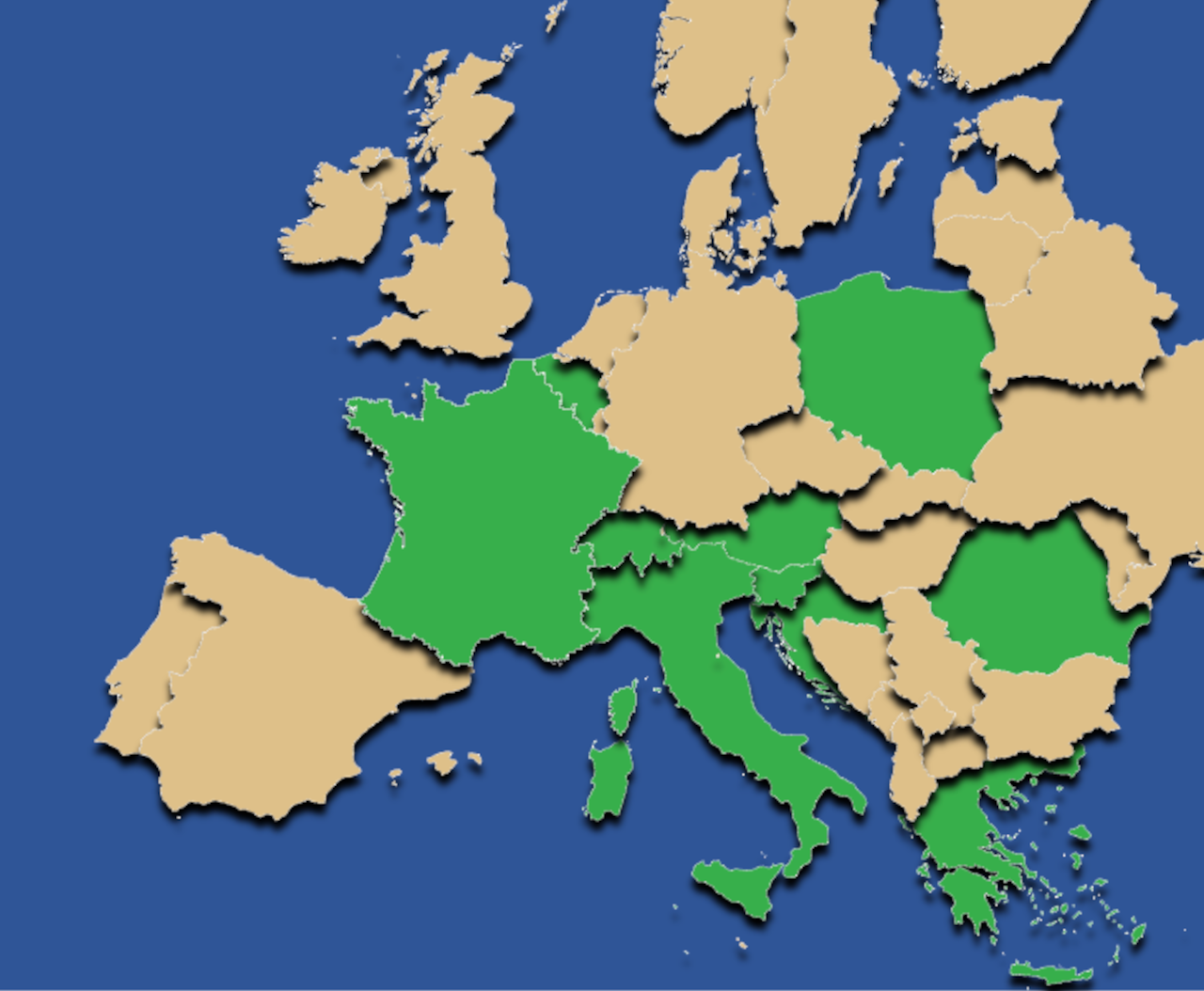
Multi-country multilingual PROs
OUR COMMITMENT TO SERVICE QUALITY

REGULATORY COMPLIANCE
We are aware that our work affects the demanding regulatory compliance of a clinical trial and we are committed to adhere strictly to all relevant compliance requirements, from FDA and EMA guidance for Good Clinical Practice (GCP) to EU-GDPR legislation for data protection. Our documented SOPs are designed with compliance requirements in mind and our team is trained to ensure compliance in all aspects of our work.

STANDARDS
Applying technical and organizational industry standards has always been one of our most fundamental work principles. We can provide clinical trial data following the CDISC family of standards, from CDASH for data collection to SDTM for tabulations and ADAM for analysis. We prepare our statistical reports from SAPs to CSRs following the ICH E3 guidelines and our clinical data management SOPs implement the SCDM guidelines.

QUALITY & INFORMATION SECURITY
Our SOPs are part of an integrated ISO 9001 / ISO 27001 certified Quality and Information Security Management System which have been designed with GCP compliance in mind, specifically for the quality and information security requirements of clinical research, covering Cloud Software Services and clinical data management work, statistical analysis and statistical programming and software development, with particular emphasis on risk management.
ABOUT AGILIS
OUR ACHIEVEMENTS
We have worked with specialized CROs, sponsors, including most major multinational pharmaceuticals and innovative biotechs exploring new drugs, as well as with research institutes and academia, and international organizations and government bodies. We have provided technology, statistics and data management from small-scale but complex Phase I and II clinical studies, to demanding Phase III and meta-analyses for regulatory submission, to large scale, multi-centre, multi-country multilingual post marketing trials and patient registries.








